An alkaline electrolyzer (also known as an alkaline water electrolysis (AWE) system) is a type of electrochemical device used to split water into hydrogen (H₂) and oxygen (O₂) through electrolysis in an alkaline electrolyte solution. This technology has been widely used for hydrogen production due to its reliability, long lifespan, and relatively low cost compared to other electrolyzer technologies, such as proton exchange membrane (PEM) electrolysis.

1. Working Principle of AWE
Alkaline electrolyzers operate based on the principle of water electrolysis, which involves the decomposition of water molecules using electrical energy. The system consists of an electrolyte solution, electrodes, a diaphragm (separator), and an external power supply.
At the cathode (-, hydrogen evolution reaction, HER):
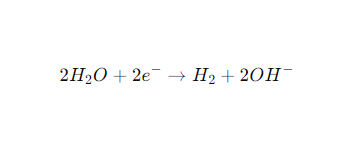
At the anode (+, oxygen evolution reaction, OER):
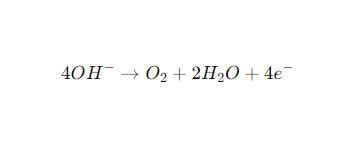
The overall response is:
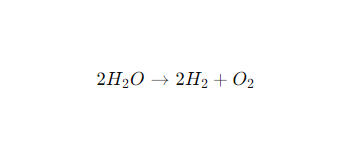
This means that for every two molecules of water, the system produces two molecules of hydrogen gas and one molecule of oxygen gas.
2. Key Components of an Alkaline Electrolyzer
a. Electrolyte (Alkaline Solution)
The electrolyte is a concentrated potassium hydroxide (KOH, 20-40%) or sodium hydroxide (NaOH, 20-40%) solution.
It serves as a medium for ion transport, ensuring that hydroxide ions (OH⁻) move between electrodes.
b. Electrodes (Anode and Cathode)
The electrodes are typically made of nickel-based materials or other corrosion-resistant metals.
The cathode (where hydrogen is produced) is often coated with nickel, iron, or cobalt-based catalysts to enhance efficiency.
The anode (where oxygen is produced) is usually made of nickel-coated stainless steel or coated with materials like manganese or cobalt oxides for better oxidation performance.
c. Diaphragm (Separator)
A porous asbestos, polymer, or ceramic diaphragm separates the anode and cathode chambers.
It allows the movement of hydroxide ions (OH⁻) while preventing the mixing of hydrogen and oxygen gases to ensure safety.
d. Power Supply
A direct current (DC) power source provides the energy required for water splitting.
The voltage applied across the electrodes typically ranges from 1.8V to 2.4V per cell, depending on operating conditions.
3. Advantages of Alkaline Electrolyzers
✅ a. Mature and Well-Established Technology
Alkaline electrolysis has been used in industrial hydrogen production for over a century.
It is widely applied in industries such as fertilizer production, metal processing, and chemical manufacturing.
✅ b. Lower Cost Compared to PEM Electrolyzers
Uses inexpensive and widely available materials (e.g., nickel electrodes instead of precious metals like platinum or iridium).
No need for expensive proton-conducting membranes.
✅ c. Long Operational Lifespan
Can run for tens of thousands of hours with proper maintenance.
Stable operation in continuous hydrogen production applications.
✅ d. Scalable for Large-Scale Hydrogen Production
Suitable for industrial-scale applications due to its ability to operate in large stacks.
